Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Lesions (cancerous) Terminology
Authors: Dr Jeffrey Lee, Medical Registrar, Christchurch Hospital, Te Whatu Ora (Health New Zealand) Waitaha Canterbury; Associate Professor Amanda Oakley, Dermatologist, Waikato Hospital, Te Whatu Ora Waikato; and Dr Michelle Vaughan, Medical Oncologist, Christchurch Hospital, Te Whatu Ora Health Waitaha Canterbury (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
The common terminology criteria for adverse events (CTCAE) describes a grading system for adverse event reporting associated with cancer therapy.
The CTCAE were developed in 1982 by the US National Cancer Institute (NCI) to collect standardised treatment-related data about adverse events to help evaluate new cancer therapies.
The CTCAE are widely used by oncologists, other internal medicine specialists, and researchers involved in cancer therapy clinical trials.
In oncology, there are a host of new active therapies including targeted agents and immunotherapy which cause skin toxicity. Dose delays and reductions, or in the case of immunotherapy, high-dose steroids to ‘turn off’ the immune response, may influence the effectiveness of the oncology agent. The CTCAE provides a framework to ensure dose changes are done according to international recommendations.
Cutaneous adverse events are found in three sections of the CTCAE:
The CTCAE are classified as Grades 1 to 5:
Body surface area (BSA) has been incorporated into grading dermatological adverse events:
Not all grades are appropriate for a particular adverse event, for example:
Some examples of cutaneous adverse events from CTCAE are listed below.
A single dash (-) indicates a definition is not available.
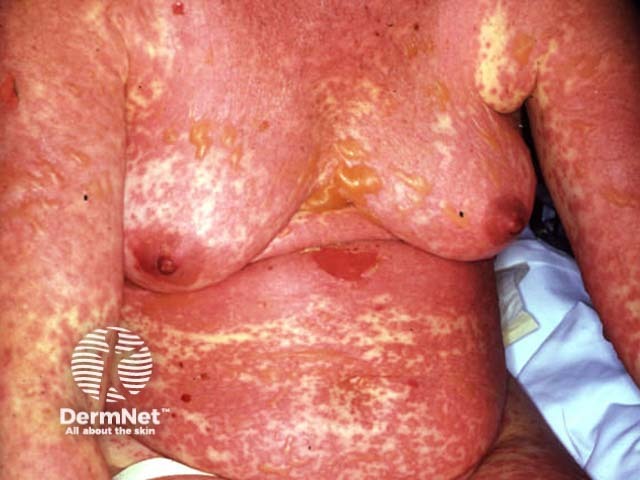
Grade 4 Stevens-Johnson syndrome / toxic epidermal necrolysis
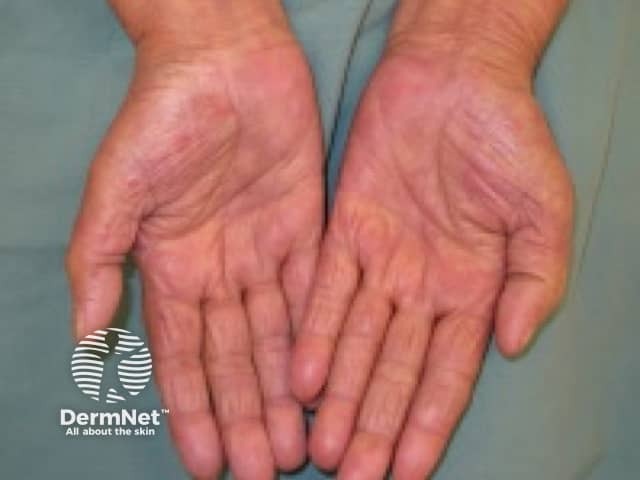
Grade 1 hand-foot syndrome
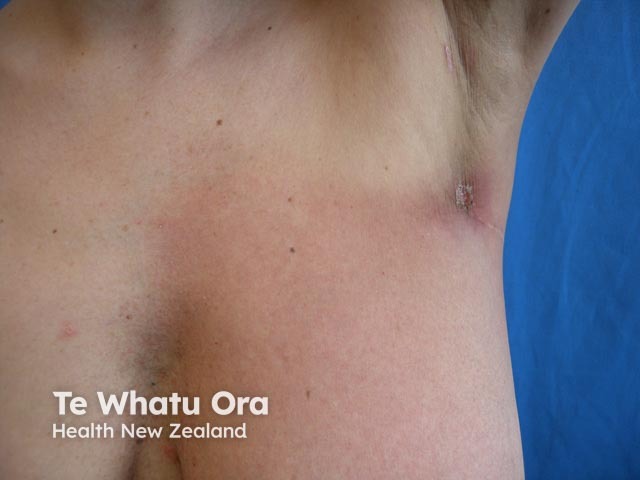
Grade 1 radiation dermatitis on the breast