Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Lesions (cancerous) Systemic diseases
Authors: Vanessa Ngan, Staff Writer, 2005; Updated: Dr Wouter Kalle, Geneticist, Charles Sturt University, Wagga Wagga, Australia. Copy edited by Gus Mitchell. July 2021
Introduction
Demographics
Causes
Clinical features
Complications
Diagnosis
Differential diagnoses
Treatment
Outcome
Lynch syndrome (OMIM 120435) is the most common inherited syndrome that predisposes to cancer. It is also known as hereditary non-polyposis colorectal cancer (HNPCC), of which Muir-Torre syndrome (OMIM 15832) is a rare specific variant.
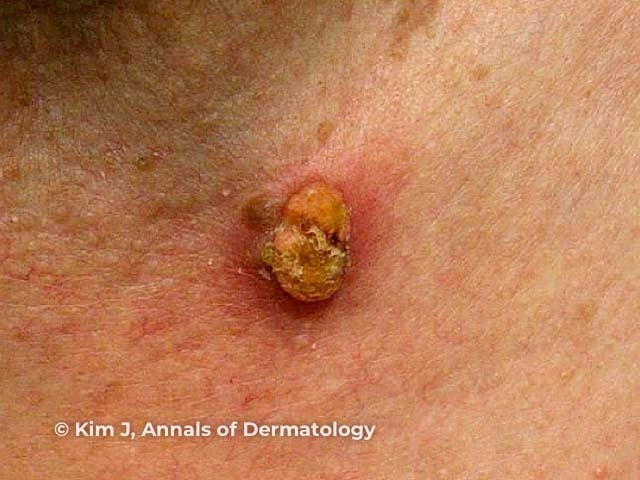
Figure 1
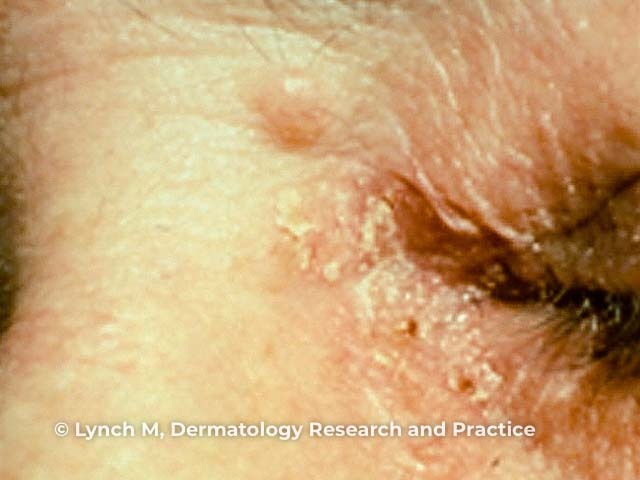
Figure 2
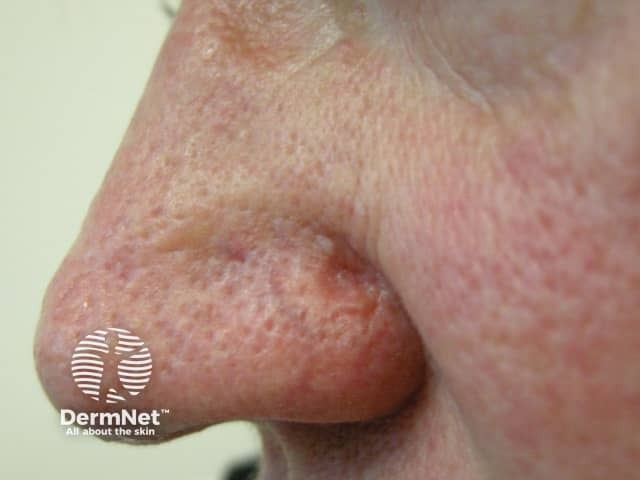
Figure 3
Figure 1 Reproduced from: Kim JE, Kim JH, Chung KY, Yoon JS, Roh MR. Clinical features and association with visceral malignancy in 80 patients with sebaceous neoplasms. Ann Dermatol. 2019;31(1):14–21.
Figure 2 Reproduced from: Lynch MC, Anderson BE. Ileocecal adenocarcinoma and ureteral transitional cell carcinoma with multiple sebaceous tumors and keratoacanthomas in a case of Muir-Torre syndrome. Dermatol Res Pract. 2010;2010:173160.
Lynch syndrome affects one in 350 individuals, including white, Asian, and African populations.
Muir-Torre syndrome (MTS) is more commonly reported in males (3:2) with an average age of onset of skin manifestations being 53 years (range 21–88 years).
Ultraviolet radiation, radiotherapy, and drug-induced immunosuppression (particularly tacrolimus and ciclosporin), such as in transplant recipients, may unmask latent Muir-Torre syndrome.
Lynch syndrome is caused by impaired DNA mismatch repair (MMR), leading to DNA microsatellite instability (MSI) and the accumulation of DNA mutations in oncogenes and tumour suppressor genes, ultimately leading to cancer. The genes involved in human mismatch repair are MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2.
Germline mutations in any one of these genes may result in Lynch syndrome, although the spectrum of malignancies may be different for each gene.
Muir-Torre syndrome is currently described as a phenotypic variant of Lynch syndrome, representing 1–2% of cases. It is usually due to mutations in the MSH2 gene (90%). MLH1 and MSH6 gene mutations have been reported but are rare in MTS. An autosomal recessive variant with microsatellite stability has been identified.
Turcot syndrome is another variant of Lynch syndrome, presenting as colonic adenomas and tumours of the central nervous system, with mutations mainly found in MLH1 and PMS2 genes.
Lynch syndrome is associated with a spectrum of malignancies including colorectal, stomach, pancreas, endometrium, ovary, urological (renal pelvis, ureter, prostate), and brain. Numerous skin tumours are described in Lynch syndrome, particularly in the Muir-Torre variant. Skin lesions may be one of the first signs of Lynch syndrome and represent 3–5% of the extracolonic cancers.
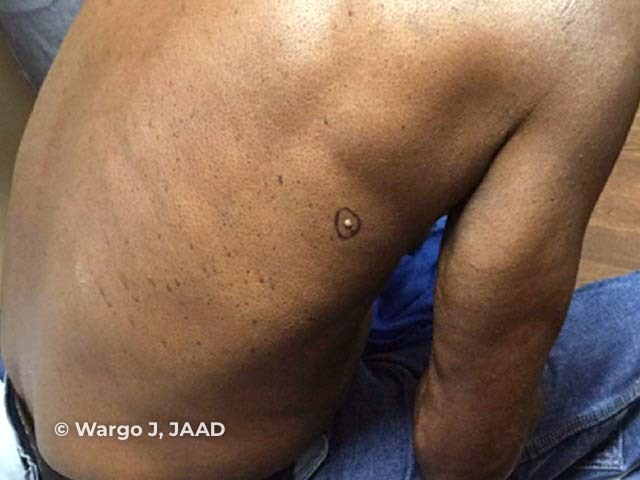
Figure 4
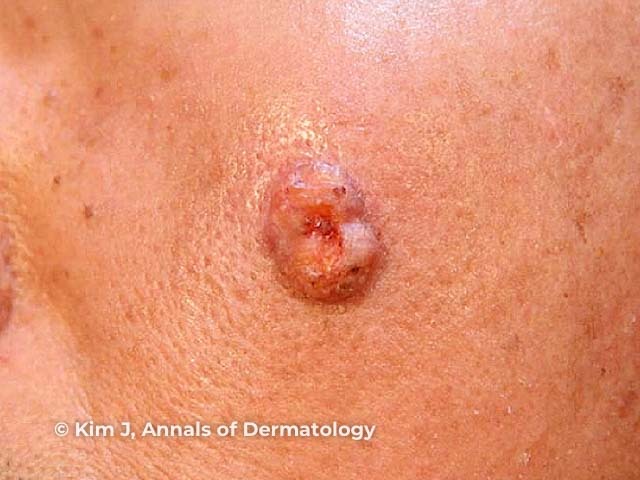
Figure 5
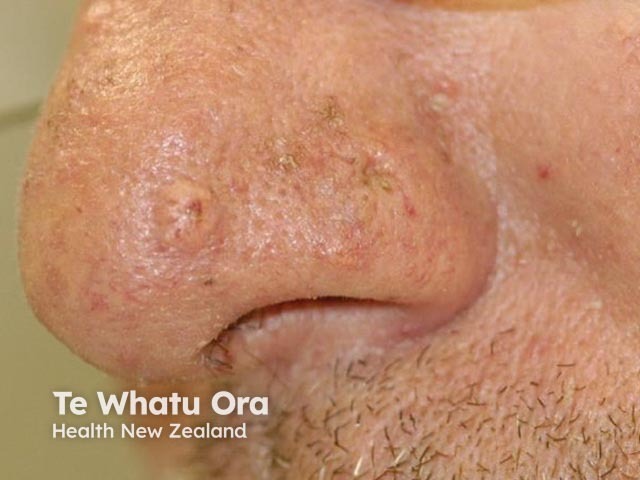
Figure 6
Figure 4 Reproduced from: Wargo JJ, Plaza JA, Carr D. Sebaceous neoplasia leading to the diagnosis of Muir-Torre syndrome in an African American man. JAAD Case Rep. 2021;11:72-3.
Figure 5 Reproduced from: Kim JE, Kim JH, Chung KY, Yoon JS, Roh MR. Clinical features and association with visceral malignancy in 80 patients with sebaceous neoplasms. Ann Dermatol. 2019;31(1):14–21.
Lynch syndrome is characterised by the development of multiple malignancies, often at a young age, which can metastasise and may be fatal. Sebaceous carcinomas can be locally invasive and metastasise.
Clinical diagnosis of Lynch syndrome is based on Amsterdam II or Revised Bethesda Guidelines and confirmed on genetic testing.
Amsterdam II Criteria:
Muir-Torre syndrome is defined by the constellation of sebaceous tumours, multiple keratoacanthomas, and other Lynch-associated malignancies. A detailed family history including first and second-degree relatives should be taken. Sebaceous tumours are diagnosed on skin biopsy [see Sebaceoma pathology, Sebaceous carcinoma pathology]. MTS should be considered in anyone under 50 years of age with a solitary sebaceous adenoma, multiple sebaceous tumours, or a sebaceous tumour not on the head and neck, and investigated initially by tissue immunohistochemistry.
Investigations may include:
Genetic testing of family members may identify individuals at risk of Lynch syndrome and therefore requiring appropriate surveillance.
Patients with Lynch syndrome require regular surveillance for Lynch-associated malignancies such as GIT endoscopy, urine cytology, imaging, and others. The age at which specific screening begins is guided by family history. It is recommended colonoscopy be performed every 1–2 years starting at age 20–25 years or 2–5 years before the earliest colorectal cancer in the family. Any cancers found should be treated appropriately eg, surgery, radiotherapy, chemotherapy, and/or immunotherapy.
Aspirin 600mg/d has been reported to decrease the risk of colorectal cancer in Lynch syndrome. Sirolimus has been recommended for patients with MTS requiring immunosuppression after a transplant.
Annual skin examination should be performed with excisional biopsy of suspicious lesions. Keratoacanthoma-like lesions should be fully excised and not watched in the expectation of spontaneous resolution. Sebaceous carcinoma requires Mohs micrographic surgery or wide local excision, with careful evaluation and follow-up of lymph nodes.
Patients with Lynch syndrome should be educated regarding symptoms and signs that may indicate malignancy such as for ovarian or endometrial cancer.
Early detection and treatment of cancers in patients with Lynch syndrome improves survival. The internal malignancies may behave in a less aggressive manner than would be predicted based on their histology. Sebaceous carcinoma has a local or metastatic recurrence rate of up to 25%.