Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, 1997. Updated by Dr Marius Rademaker, Dermatologist, Hamilton, New Zealand; Dr Monisha Gupta; Dr John Sullivan, December 2015.
Introduction
Indications
Onset of improvement
Mechanism of action
Contraindications and precautions
Administration
Tests prior to initiation
Side-effects
Drug interactions
Vaccination
Safety measures
Methotrexate is a medication used in low doses to treat inflammatory skin conditions such as psoriasis and eczema/dermatitis. It is also prescribed for rheumatoid arthritis, psoriatic arthritis, and increasingly, other inflammatory and autoimmune disorders (off-label). In much higher doses, it is used as a chemotherapy agent for leukaemia and some other forms of cancer.
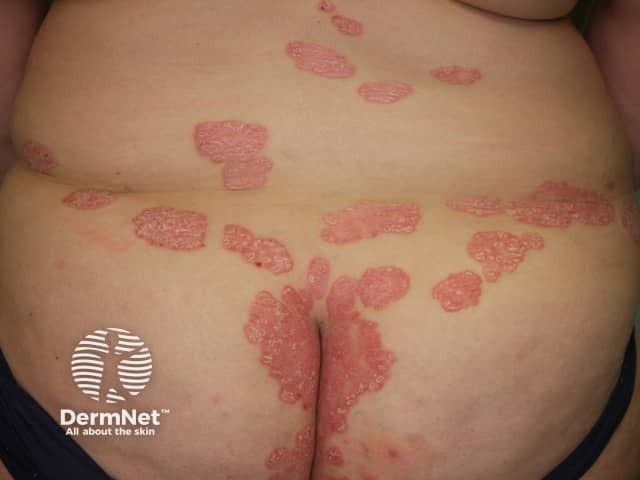
Psoriasis
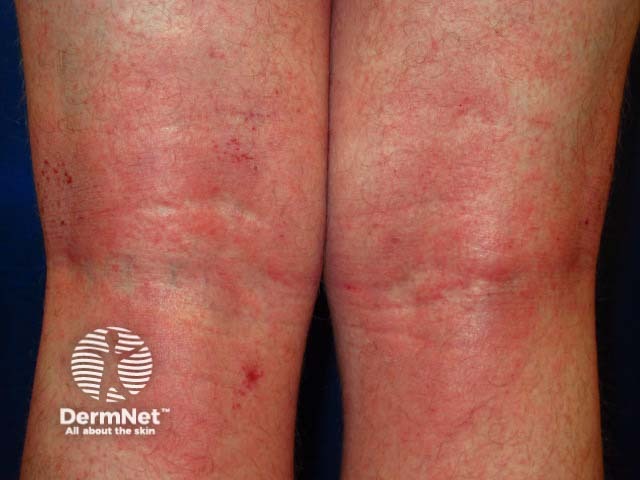
Atopic eczema
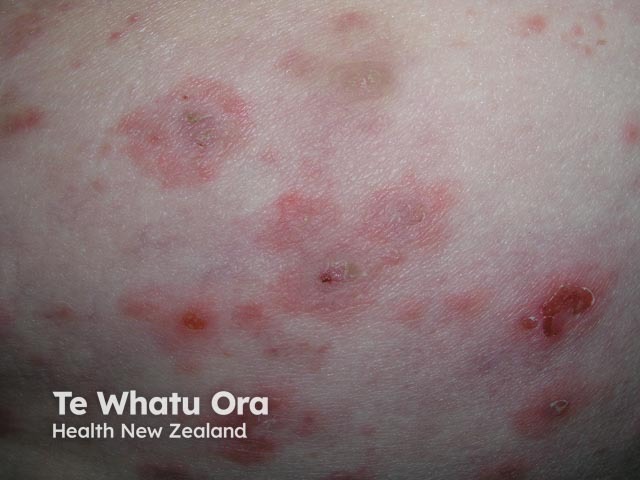
Bullous pemphigoid
Methotrexate has been used in the treatment of moderate to severe psoriasis for many years. It is now used in suitable patients that have other extensive or troublesome skin conditions. These include:
Methotrexate is used in adults and in children over 3 years of age.
Methotrexate usually shows some benefit in responding skin diseases within 6 to 8 weeks. Maximum effects are generally achieved within 5 to 6 months, depending on dose escalation. In chronic plaque psoriasis, about 50–70% of patients see a good result (a reduction in PASI score of 75%). To measure a PASI score, see also Patient-oriented psoriasis PO-PASI score.
There are several mechanisms that may explain the effect of low-dose methotrexate in skin diseases.
Methotrexate is Australian Therapeutic Goods Administration (TGA) and US Food and Drugs Administration (FDA) pregnancy category X. At high dosages, methotrexate is known to cause miscarriage or stillbirth, especially in the first 3 months of pregnancy. There is also a concern regards the risk of lower dosage methotrexate affecting functional development in the later stages of pregnancy. It is therefore recommended that pregnant women do not take methotrexate, and women of childbearing age should not become pregnant while taking methotrexate. Adequate contraceptive measures are necessary during therapy. Consult your doctor before considering pregnancy.
The amount of methotrexate excreted in breast milk is likely to be small. It is unclear how much would be absorbed by a nursing baby. However, the precautionary approach is to limit or avoid breastfeeding in mothers being treated with methotrexate.
For many years men were advised not to father children while they were on methotrexate and for at least 3 months afterwards because methotrexate had been reported to cause a reduction in sperm count. The current expert view is that the risks are very low, and this precautionary approach is not necessary.
Methotrexate is contraindicated in the presence of serious acute or chronic infection. Therapy should be withheld or discontinued until the infection has resolved, as appropriate, taking into account the clinical indication for methotrexate, the availability of alternative treatments, and the severity of the infection.
For patients with non-serious active infection, methotrexate should be used with caution.
Methotrexate should be taken with caution by patients with mild to moderate liver or kidney disease, low blood counts (anaemia, leukopenia, thrombocytopenia), obesity, or diabetes.
Methotrexate is contraindicated in patients with severe liver or kidney disease.
Dehydration from fever, vomiting, diarrhoea, or decreased fluid intake may increase levels of methotrexate. Excessive thirst may be a symptom of dehydration. Notify your doctor if these symptoms develop before you take the next dose of methotrexate.
Dehydration or any other reason for reduced kidney function may prevent normal excretion of methotrexate resulting in toxic accumulation of the medication. Excessive methotrexate can in turn damage the kidneys further.
Alcoholic beverages (including beer and wine) may increase some of the side effects, including the chance of liver damage, and should be restricted to less than 20 g/day (1–2 standard drinks/day; 10–14 drinks/week).
See guidelines of The Alcohol Drug Association of New Zealand, The Alcohol Advisory Council of New Zealand (ALAC) on safe drinking.
The expert view is that methotrexate does not need to be stopped for any surgery.
Methotrexate is available as 2.5 mg and 10 mg tablets, and as a solution for injection. Take care not to mix up the 2.5 mg and 10 mg-sized tablets — you might end up taking an overdose, or too little.
Most people are prescribed tablets. The most common dose is 15 mg each week, but it varies from 2.5 mg to 30 mg each week depending on kidney function, side effects experienced, and efficacy in treating the skin disease. The doctor may decide to start with a very low dose such as 2.5 to 5 mg and then gradually build it up to the full dose over several weeks. An initial lower dose should be used if there is reduced renal function (kidney disease), in patients with lower body weight, or in older patients (> 75 years of age).
Methotrexate is taken weekly, rather than daily.
This is different from most medications. The importance of this weekly schedule cannot be stressed enough; it is best if a specific day of the week is nominated (eg Monday) on the prescription. On the specified day of the week, the methotrexate can be taken either as a single dose or split into 2 to 3 smaller doses.
If methotrexate tablets cause nausea, your doctor may recommend splitting the dose, taking it after meals or at bedtime two days a week.
If the oral treatment causes too many gastrointestinal side effects, a once-weekly subcutaneous injection may be tried. This is generally well tolerated. One study reported PASI 75 (ie, 75% reduction in initial PASI score) at week 16 in 41% of patients treated with 17.5 to 22.5 mg/week.
Taking methotrexate more often, or changing the dosing schedule may result in serious side effects. If doses are taken too often, notify your doctor at once.
Pre-treatment laboratory tests usually include a full blood count with differential (CBC), kidney function tests (creatinine), liver function tests, HbA1c (a test for diabetes), and lipids.
Hepatitis B and C serology, HIV and varicella (chickenpox) serology and tuberculosis testing (QuantiFERON®-TB Gold) may be considered in populations at risk of these infections. Pretreatment FibroScan®/liver biopsy is rarely necessary but may be considered if there is an existing liver disease or high risk of liver disease.
Consider measuring trough level polyglutamated methotrexate as a measure of 1) compliance and 2) whether a dose adjustment is necessary.
In some regions, a blood test measuring type 3 procollagen amino-terminal propeptide (P3NP collagen) may be requested in adults aged 20–70 years before treatment with methotrexate and repeated every 3 to 6 months while on methotrexate.
P3NP collagen measurement can be used to assess hepatic fibrosis in patients with psoriasis on long term methotrexate. Three elevated levels over a 1-year period may indicate liver damage. P3NP may be elevated in a variety of conditions, including:
P3NP testing in childhood is less reliable, as normal growth leads to higher P3NP collagen levels.
A liver ultrasound scan using transient elastography (FibroScan®) measures the stiffness of the liver and may reveal fibrosis or cirrhosis. If the result is in the normal range, it is very unlikely that methotrexate is causing liver fibrosis (i.e a normal scan is a negative predictor of liver damage).
It may occasionally be necessary to take a small specimen of liver tissue with a needle (liver biopsy). Liver biopsy findings may be reported using Roenigk classification:
A chest X-ray is recommended in anyone with a recent or past history of lung disease. Medsafe in New Zealand recommends that all patients prescribed methotrexate have a baseline chest X-ray and that it is repeated should they develop respiratory symptoms.
Side effects can occur at any time during treatment with methotrexate but are most common in the first few weeks. Folic acid supplements may be prescribed, as they are thought to reduce some of the side effects of methotrexate. There is still some debate as to the best dose and timing of folic acid. The current expert recommendation is to take 5 mg once a week, eg on a Friday (Monday for methotrexate, Friday for folic acid).
If the side effects described below or other problems trouble you, or should you develop any signs of infection or unusual bleeding, notify your doctor promptly and before your next dose of methotrexate is due.
The most common side effects of methotrexate are loss of appetite, nausea and diarrhoea, and affect about one in 12 patients. These side effects are usually temporary, but changes in dose and/or supplemental folic acid tablets may be helpful. If you have gastroenteritis (stomach upset), do not take methotrexate until you have recovered. Mouth ulcers or diffuse stomatitis are uncommon at normal dermatological doses.
An overdose of methotrexate or deficiency of the vitamin folic acid may result in anaemia (decreased haemoglobin), leucopenia (reduced white cell count, risking serious infections), and thrombocytopenia (low platelet count, resulting in bruising and bleeding). Methotrexate should not be taken unless the blood count is normal or near-normal prior to the next dose. Low blood counts are more likely in people with kidney disease, with existing haematological disorders, or when taking other medications (particularly sulfonamides).
Methotrexate is stored by the liver. Transaminase liver enzyme levels may rise for a few days after treatment but they quickly return to normal. It's best to do blood tests at least 5 days after a dose, or just prior to the next dose.
Long term therapy may be associated with scarring (fibrosis or cirrhosis) of the liver. This is more commonly due to other reasons such as fatty liver, diabetes, hyperlipidaemia, and obesity (ie metabolic syndrome), but can also develop from viral hepatitis and alcohol.
Methotrexate can rarely cause a lung reaction similar to pneumonia called acute pneumonitis or interstitial pneumonia. The symptoms are usually fever, cough (often dry and hacking), and shortness of breath. Should you develop such symptoms, stop taking methotrexate and notify your doctor promptly. A chest X-ray may reveal diffuse white patches.
Slowly progressive lung fibrosis or bronchiolitis obliterans associated with methotrexate is rare. Recent studies have not shown this a risk in patients receiving methotrexate for dermatomyositis; in some variants the disease itself may be associated with interstitial lung fibrosis. Eosinophilic pneumonia is also reported. As with acute pneumonitis, chronic lung disease usually affects patients with rheumatoid arthritis on methotrexate.
Although uncommon, methotrexate may rarely result in reactivation of tuberculosis or opportunistic bacterial, fungal or viral infections. Shingles (herpes zoster infection) and cold sores (herpes simplex) may be more severe in those taking methotrexate. Methotrexate is contraindicated in patients with significant immunodeficiency, untreated tuberculosis, untreated HIV infection, or other serious active infections.
If an accidental overdose occurs, folinic acid injections may be necessary. The antidote should be given as early as possible.
Methotrexate may rarely cause skin problems.
Some patients taking methotrexate have headaches, dizziness, fatigue and mood changes, especially when first starting on methotrexate. Nausea, confusion or headache can also rarely be due to hyponatraemia (sodium imbalance).
Refer to datasheets and prescribing information for a full list of risks and side effects.
Several medications may increase side effects or decrease the effectiveness of methotrexate or the other drug. These are all unlikely with low-dose methotrexate.
Tell your doctor all the medicines you are taking, whether they are prescription or non-prescription medicines. If you are having an operation with a general anaesthetic, tell the anaesthetist you are on methotrexate. It is not necessary to stop methotrexate for an operation, but always discuss this with your surgeon.
Do not begin or change the dosage of any medicine without first checking with your doctor. This is especially true of antibiotics and anti-inflammatory agents.
Like methotrexate, antibiotics that contain the drug trimethoprim or sulfonamides (eg trimethoprim + sulphamethoxazole) antagonise folate. Taking them at the same time as methotrexate could increase the toxicity. Penicillins, minocycline and ciprofloxacin may also increase methotrexate toxicity.
Aspirin and aspirin-like drugs (nonsteroidal anti-inflammatories) may reduce how much methotrexate is eliminated by the kidneys. This could potentially result in a toxic build-up of methotrexate in the bloodstream. Anti-inflammatories can often be taken safely but you should have regular blood tests if you start these medicines or others, as advised by your doctor. Alternatively, you could take paracetamol (acetaminophen), as this does not interfere with methotrexate. Do not take excessive paracetamol, as this can cause liver damage.
Other drugs that may increase methotrexate toxicity include barbiturates, proton pump inhibitors (pantoprazole, omeprazole, esomeprazole, lansoprazole, rabeprazole), colchicine, dipyridamole, phenytoin, sulfonylureas, frusemide/furosemide and thiazide diuretics. Ask your doctor's advice if you take any of these medicines.
Vaccines (live and/or killed) may be less effective in those taking methotrexate, so it is best if you are fully immunised before starting methotrexate. If this is not possible, a double dose of the vaccine may be required.
Killed vaccines are safe and are often advised to reduce the impact of infection — arrange annual influenza vaccination.
There is an on-going debate as to the risks of live vaccination in patients taking methotrexate. Many dermatologists believe the benefits of vaccination far outweigh the increased risk, with the possible exception of yellow fever vaccination. Discuss the vaccination with your doctor or travel physician. See Immunisation in immunosuppressed dermatology patients.
Methotrexate should be kept out of the reach of children. Do not give this medication to other people. Dispose of the injected form of methotrexate in appropriate sharps containers.
Close monitoring, ie medical supervision of patients on methotrexate is essential. It is important that you carry out your doctor's instructions faithfully and promptly report any side effects or symptoms you may develop.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).