Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: January, 2024
Authors: Dr Hyun Kyoung Lee, Medical Registrar, Middlemore Hospital; and Honorary Associate Professor Paul Jarrett, Dermatologist, Middlemore Hospital and Department of Medicine, The University of Auckland, Auckland, New Zealand (2024)
Previous contributors: Dr Marie Hartley, Staff Writer (2010)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Sulfonamide antibiotics
Sulfa allergy
Non-antibiotic sulfonamides
Cross-reactivity
Other sulfur drugs
‘Sulfa’ is a broad term used to describe medications that contain a sulfonamide (sulphonamide) functional group within their molecular structure. They can be categorised into sulfonamide antimicrobials and sulfonamide non-antimicrobials.
The term ‘sulfa’ may be confused with other drugs and products with names stemming from ‘sulfur’, although they have different meanings. Allergies to sulfates, sulfites, or sulfhydryl bear no relationship to sulfonamide allergy.
Sulfonamide antibiotics have the highest incidence of drug reaction compared to other antibiotics, with approximately 3–8% of those exposed having an adverse reaction.
Cutaneous reactions are the most common adverse event associated with sulfonamide drugs, occurring in 1.5–3% of immunocompetent patients. However, the most significant risk factor for sulfonamide allergy is HIV positivity, with an incidence of approximately 27% associated with the use of trimethoprim and sulfamethoxazole (TMP-SMX) to treat pneumocystis jirovecii pneumonia (PJP).
There are currently no validated tests available to determine the risk of sulfonamide allergy. Skin prick testing has limited value as type 1 allergic reactions are less common.
Sulfonamide antimicrobials contain additional groups within their chemical structure which are usually absent in non-antimicrobial sulfonamides including: N4 aryl-amine and a N1 heterocyclic ring. These additional groups are mainly responsible for reactions to sulfonamide antimicrobials rather than the sulfonamide functional group.
Type 1 immunoglobulin E (IgE)-mediated reactions may occur as the IgE antibody binds to the N1 heterocyclic ring. Non-type 1 reactions are usually associated with metabolites of the antimicrobial agents.
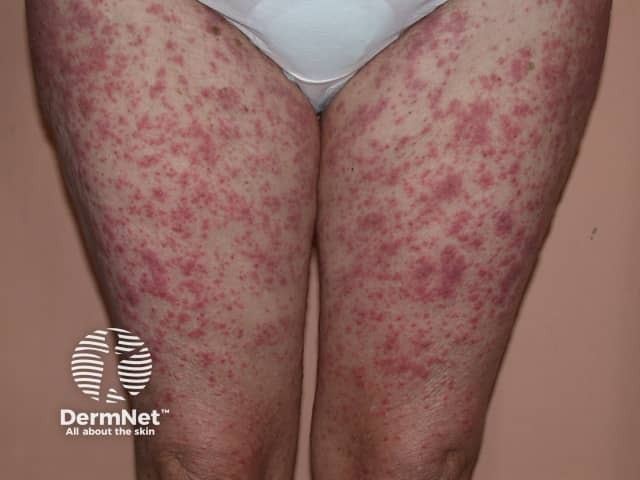
An exanthematous sulfonamide reaction
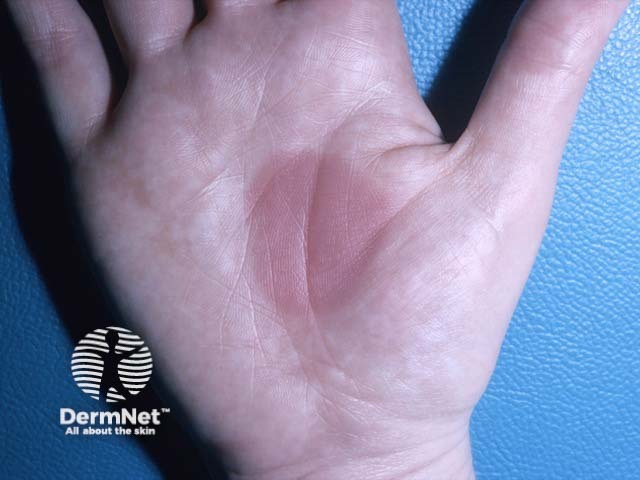
A fixed eruption - the rash recurred on every exposure to sulfonamides in the same location (FDE-patient1)
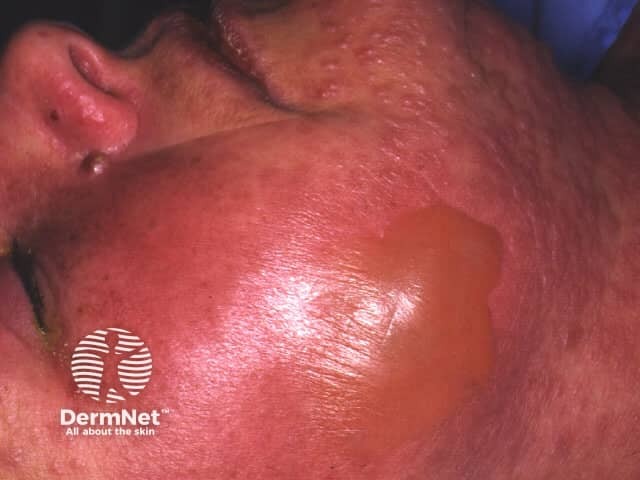
Sulfonamide-induced toxic epidermal necrolysis
A commonly used sulfonamide antibiotic is sulfamethoxazole and trimethoprim (TMP-SMX). Patients who react to this drug may be allergic to trimethoprim, sulfamethoxazole, or both. It is known that trimethoprim on its own can cause type 1 allergy (anaphylaxis) and Stevens–Johnson syndrome / toxic epidermal necrolysis. Patients known to react to TMP-SMX should avoid both trimethoprim and sulfamethoxazole.
Other examples of sulfonamide antimicrobials:
Examples of non-antimicrobial sulfonamides:
Many adverse reactions to sulfonamides are not of an allergic nature. Types of non-allergic reactions to sulfonamide antibiotics include nausea, diarrhoea, and candidiasis.
Other types of adverse reactions include:
Rarely:
All sulfonamide antimicrobials contain the additional groups of N4 aryl-amine and the N1 heterocyclic ring which are associated with sulfonamide drug allergy. This leads to cross-reactivity among sulfonamide antibiotics.
Sulfonamide non-antimicrobials do not have a N1 heterocyclic ring as this group is responsible for antimicrobial activity and most do not contain the N4 aryl-amine.
Current evidence shows there is low risk of cross-reactivity between sulfonamide antimicrobial agents and sulfonamide non-antimicrobials. However, it is important to note that patients with true sulfonamide allergies are more likely to have allergic reactions to other medications which are not due to cross-reactivity.
The term ‘sulfa drug’ refers to medications with the sulfonamide group within their molecular structure. However, this can cause some confusion as it can lead to avoidance of medications with a ‘sulf’ prefix that contain sulfur (sulphur). Medications that contain sulfur but lack the sulfonamide functional group have no risk of cross-reactivity to sulfonamide medications.
Examples of medications that have no relationship to sulfonamide allergy but contain sulfhydryl or sulfate:
Sulfhydryl-containing drugs (thiols) can cause unrelated adverse skin reactions such as a rash or drug-induced pemphigus (a rare autoimmune blistering disease).
Sulfate allergies are extremely rare.
Sulfites (eg, sodium sulfite) are used as preservatives in food and drugs. Allergy to sulfite preservatives can cause urticaria and exacerbate asthma.
People who have had allergic reactions to sulfonamide-containing drugs do not need to avoid sulfhydryl drugs, sulfates, or sulfites.
People with sulfonamide allergy may cross-react with para-aminobenzoic acid (PABA) and paraphenylenediamine (PPD).
Immediately discontinue the offending drug. Mild cutaneous reactions will self-resolve.
Topical steroids and antihistamines may be needed. For severe reactions, hospital admission and treatment for skin failure is required. Patients with SJS/TEN are best managed in a burns unit.
An alert should be placed in the patient’s notes, the patient informed and given a record of the offending medicine for future reference; consider an alert bracelet for serious reactions.
If a patient is considered to need a sulfonamide medication to which they previously reacted, rechallenge and desensitisation may be appropriate for those with a mild maculopapular eruption. However, alternatives should be considered for those with a history of severe reaction.