Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: May, 2025
Author(s): Kameron Li, The University of Auckland; Honorary Associate Professor Paul Jarrett, Dermatologist, Middlemore Hospital; Nancy Huang (MBChB), DermNet Medical Writer, New Zealand (2025)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department.
Introduction
Uses
Contraindications and precautions
More information
Benefits
Disadvantages
Side effects and risks
For information on opioid receptor agonists, see opioid receptor agonists in dermatology.
Naltrexone and naloxone are both competitive antagonists of opioid receptors, binding with high affinity to the mu (μ) opioid receptors. They also exhibit affinity for kappa (κ) and delta (δ) opioid receptors, but to a lesser extent.
Naltrexone is used to maintain abstinence in alcohol and opioid use disorders through its suppressive effects on cravings. When given in fixed combination with bupropion, naltrexone is also an FDA-approved weight loss drug called Contrave®.
There has been growing interest in the use of low-dose naltrexone (LDN) in inflammatory and chronic pain conditions. LDN refers to a dosage of around 1-5 mg daily. For comparison, the standard dose used in alcohol or opioid use disorders is 50-100 mg daily, orally.
Off-label uses for LDN include:
Naloxone is used for the rapid reversal of opioid toxicity or overdose.
At standard doses (50-100 mg/day orally):
At low doses (1-5 mg/day orally):
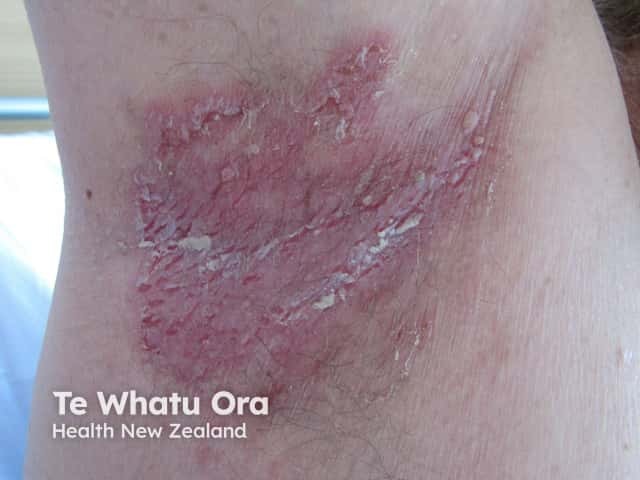
Axillary Hailey-Hailey disease - low-dose naltrexone can help this condition
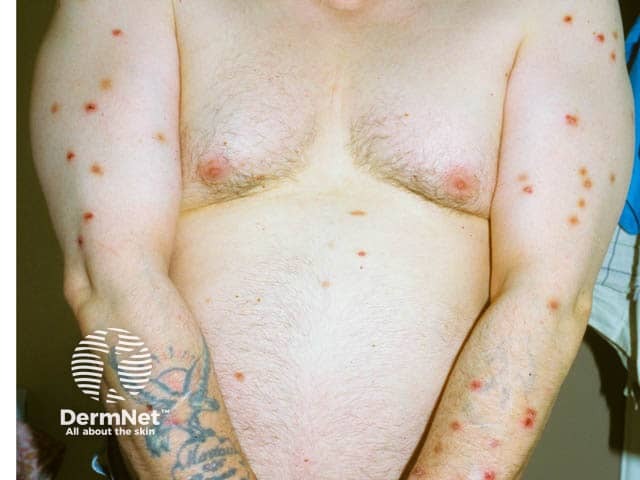
Prurigo may improve with standard dose naltrexone
There are no absolute contraindications for naloxone in emergency use. The only relative contraindication is hypersensitivity to naloxone or its excipients.
Naloxone is considered compatible for use in pregnancy and breastfeeding.
Naltrexone is typically given orally and has a half-life of 5 hours. Its active metabolite, 6β-naltrexone, has a half-life of 15 hours. The half-life of naltrexone can be extended by administering it as an implant or intramuscular injection (depot).
Naloxone is administered parenterally (intravenously, subcutaneously, or intramuscularly) or by nasal spray, and it has a short half-life of 30 to 90 minutes.
While regulatory approval for dermatological conditions is lacking, emerging evidence from case series and small trials supports their off-label use. Larger, robust clinical trials are needed to establish standardised dosing, efficacy, and long-term safety for these indications.
LDN is proposed to exert its effects through the transient blockade of opioid receptors, binding for only 4-6 hours compared to 24-48 hours with standard dosing. This intermittent blockade promotes a paradoxical upregulation in endogenous opioid production and opioid receptor expression.
Moreover, LDN also antagonises Toll-like receptor 4 (TLR4) on microglial cells, which are the primary immune cells in the central nervous system. This action inhibits the release of pro-inflammatory cytokines. Together, enhanced opioid signalling and microglial modulation produce anti-inflammatory and anti-nociceptive effects.
(For information on opioid receptors, see: opioid receptor agonists in dermatology).
In non-opioid dependent patients, serious adverse effects are rare, and most side effects are mild and transient.
Side effects of low-dose naltrexone are usually milder and fewer in number than those observed with standard doses. They include:
In rare cases, naltrexone has been reported to paradoxically exacerbate itch in cholestatic and mycosis fungoides-associated pruritus.
In the context of acute opioid reversal, there have been reports of ventricular tachycardia, atrial fibrillation, pulmonary oedema, and cardiac arrest occurring after naloxone administration in postoperative patients, most of whom had pre-existing cardiovascular disorders or received potentially cardiotoxic drugs. A causal relationship has not been established.
New Zealand approved datasheets are the official source of information for these prescription medicines, including approved uses and risk information. Check the individual New Zealand datasheet on the Medsafe website.
If you are not based in New Zealand, we suggest you refer to your national drug approval agency for further information about medicines (eg, the Australian Therapeutic Goods Administration and the US Food and Drug Administration or a national or state-approved formulary (eg, the New Zealand Formulary and New Zealand Formulary for Children and the British National Formulary and British National Formulary for Children).