Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Authors: Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand, 2001. Updated: Hana Numan, Medical Writer, New Zealand. Copy edited by Gus Mitchell. October 2021
Introduction
Demographics
Contraindications and precautions
More information
Benefits
Disadvantages
Side effects and risks
Azathioprine is an antimetabolite thiopurine analogue drug that interferes with DNA synthesis and suppresses the immune system response.
Azathioprine is metabolised in the liver to mercaptopurine, also known as 6-mercaptopurine (6-MP) which is also commercially available as a drug. Mercaptopurine is then converted into thioguanine nucleotides which inhibit cell growth.
Azathioprine is more often used in practice than mercaptopurine for the treatment of skin conditions, however they are closely related.
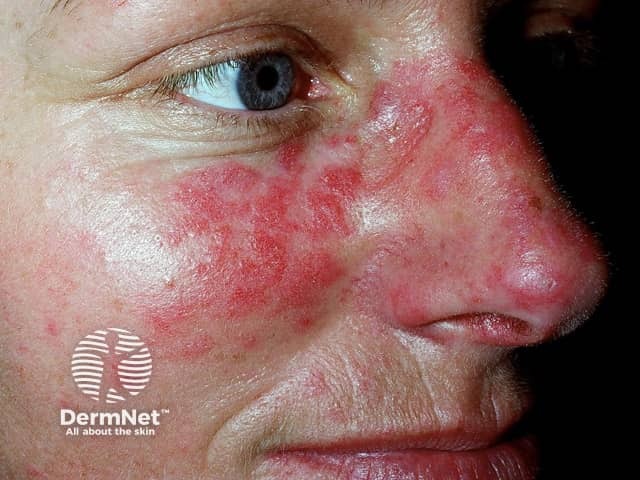
Systemic lupus erythematosus
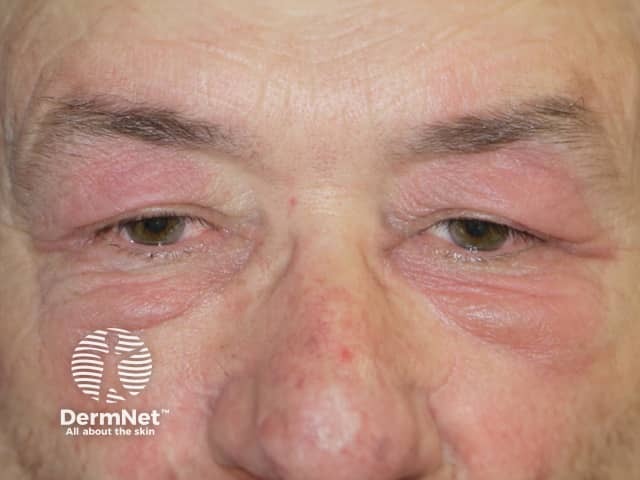
Dermatomyositis
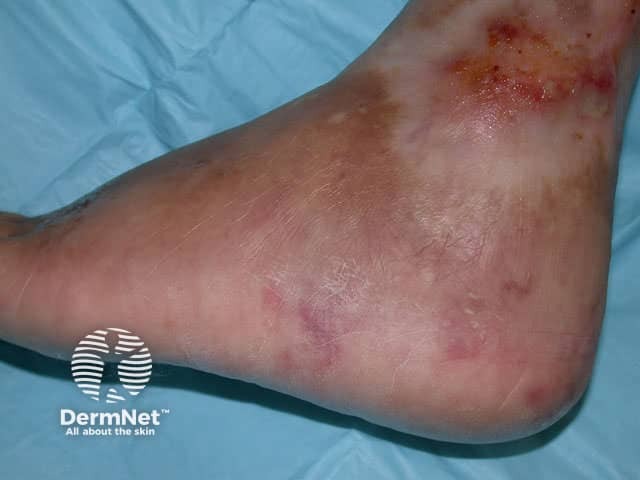
Polyarteritis nodosa
Limited evidence is available for many other dermatologic conditions.
Treatment with azathioprine and mercaptopurine during all stages of pregnancy should be avoided except where benefits outweigh risks.
Females of childbearing potential should be advised of the risks involved and use effective contraception where appropriate. Teratogenicity is unclear in men taking azathioprine or mercaptopurine and adequate contraception may also be advised in this case.
Although low concentrations of 6-mercaptopurine may be found in breastmilk, there has been no evidence to suggest harm. Potential benefits should outweigh risks; for further information, see Lactation and medications used in dermatology.
Azathioprine has been widely prescribed since the 1960s, however its mechanism of action as well as that of its metabolite 6-MP is not fully understood. There are a number of proposed mechanisms including:
Azathioprine and mercaptopurine may be available as an oral tablet, oral liquid, or intravenous injection (azathioprine only).
The dose of azathioprine is generally 1–3 mg/kg/day, however the individualised dose is dependent on several factors including indication, age, response, and TPMT activity (see below). The bioavailability may vary between different formulations. Dose reduction may be required in patients with hepatic and renal impairment.
Effects of azathioprine and mercaptopurine may take up to several months to be seen — this should be considered when reviewing treatment.
Azathioprine and mercaptopurine usually cause mild side effects but may occasionally be severe enough to stop treatment. These include:
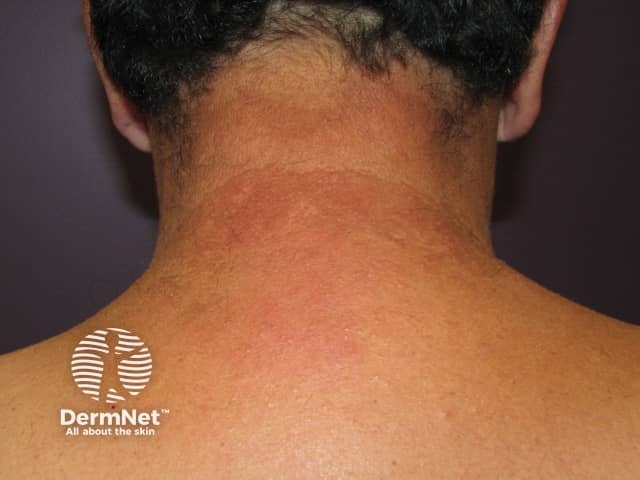
Drug-induced photosensitivity
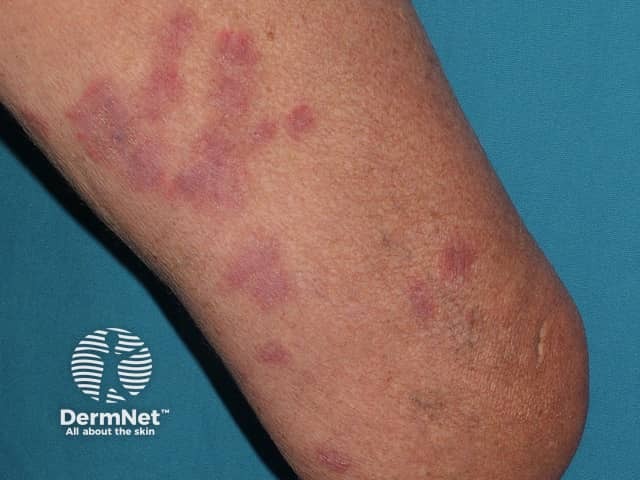
Acute febrile neutrophilic dermatosis
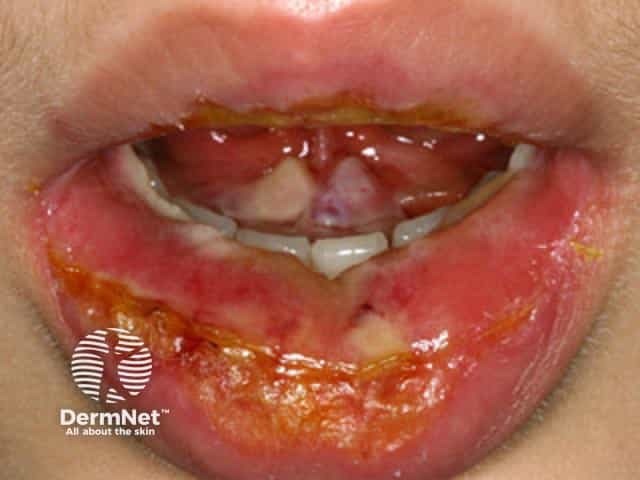
Toxic epidermal necrolysis
If the use of an interacting drug combination is unavoidable, dose adjustment may be required and blood counts should be monitored carefully. Interactions include:
Before starting treatment:
During treatment:
Azathioprine and mercaptopurine is primarily metabolised by the enzyme TPMT. Some people have a genetic mutation causing them to have either low enzyme activity (approximately 11% of the population) or a lack of enzyme activity (1 in 300 people). Those with both genes are at severe risk of bone marrow suppression. Conversely, some other individuals have high levels of enzyme activity and may require a higher dose than normal.
TPMT levels should be measured to determine a patient's level of risk before starting treatment. Low levels are < 5 U/mL, intermediate levels are 5–13.7 U/mL, and high levels are > 13.8 U/mL.
Azathioprine should be stopped and promptly managed jointly with a haematologist if:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).