Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Lesions (cancerous) Lesions (benign)
Authors: Rajan Ramji, Clinical Medical Education Fellow, Faculty of Medical and Health Sciences, The University of Auckland, Auckland, New Zealand; Jenny Chung, Dermatology Registrar, Middlemore Hospital, Auckland, New Zealand. Copy edited by Gus Mitchell. February 2022
Introduction
Demographics
Causes
Clinical features
Diagnosis
Treatment
Outcome
The scalp comprises the area from the back of the head (beginning at the superior nuchal lines) to the eyebrows (supraorbital margin). Scalp tumours are benign or malignant cutaneous lesions which arise on the scalp.
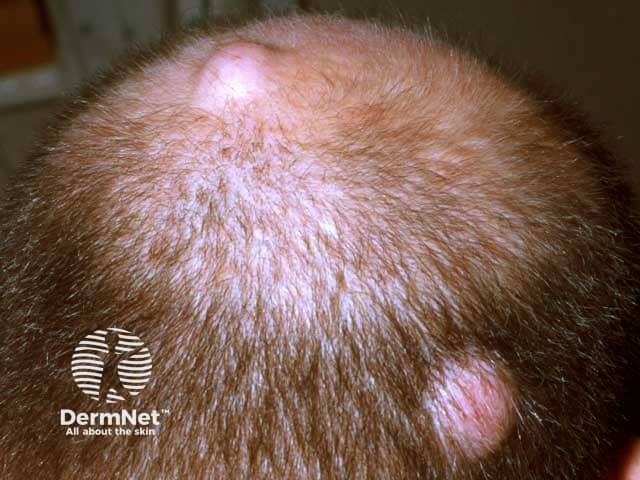
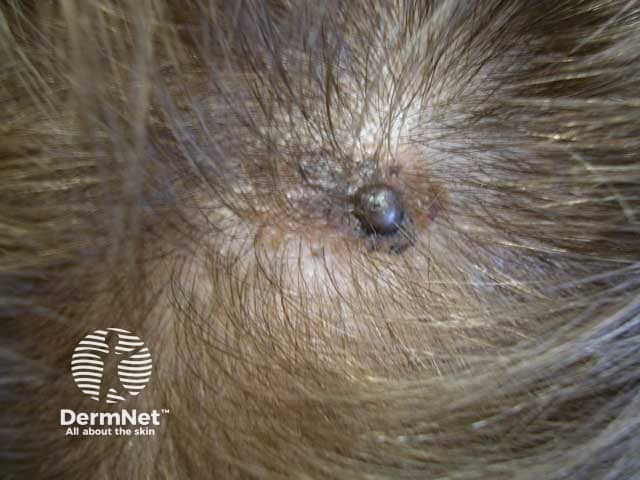
Pilar scalp cysts
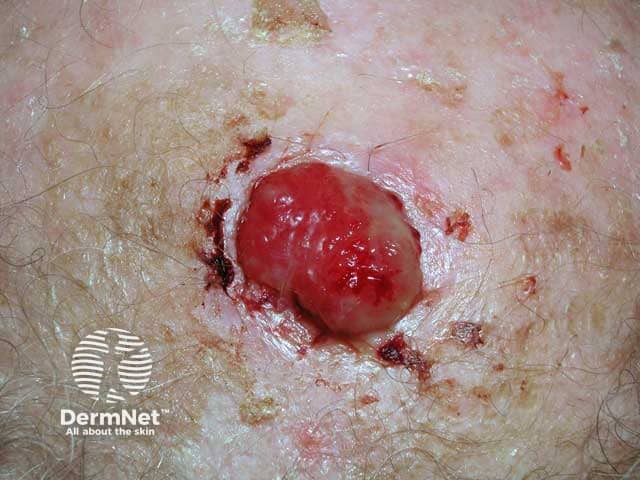
An ulcerated atypical fibroxanthoma on a bald scalp
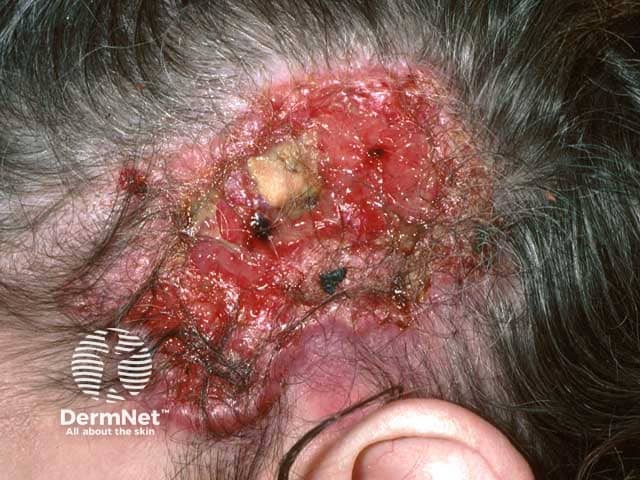
Giant neglected basal cell carcinoma ulcerated down to the skull
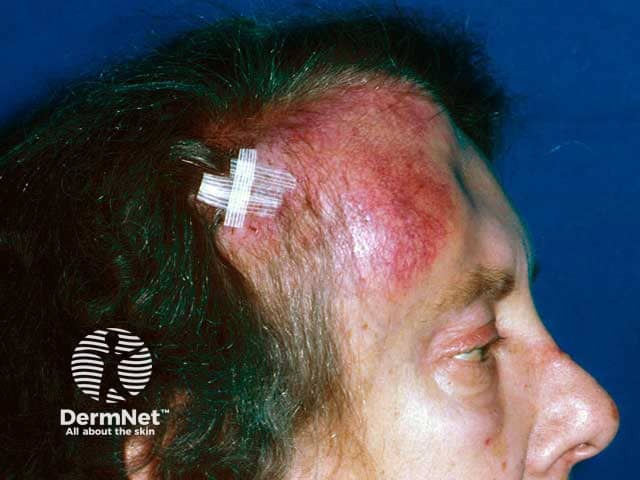
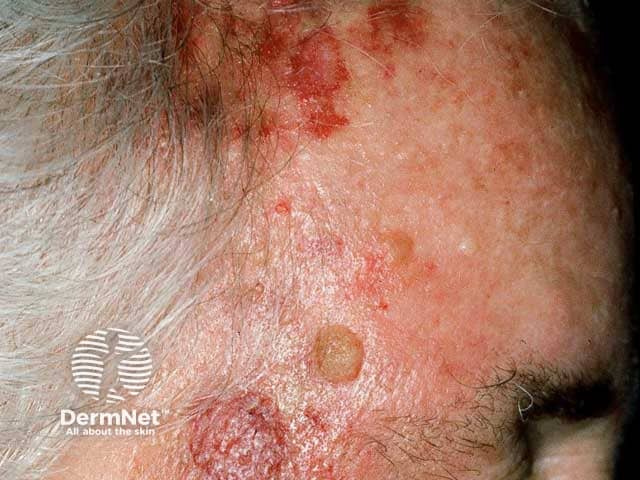
Sclap and forehead tumour due to B cell lymphoma
Superficial spreading malignant melanoma on the scalp with recent development of nodular component
Angiosarcoma
Scalp tumours occur worldwide. Most scalp tumours (93–99%) are benign as opposed to malignant.
Approximately 40–50% of benign scalp tumours are cysts with an estimated 20% incidence in Western populations. Trichilemmal (or pilar) cysts are especially common and it is estimated 80% of these cysts occur on the scalp. The remaining proportion of benign scalp tumours primarily comprises lipomas (~30%) and melanocytic naevi (28%). Seborrhoeic keratoses and actinic keratoses are increasingly common with age and the latter develop particularly as the hair thins.
Although only 1–2% of scalp tumours are malignant, they comprise approximately 13% of malignant cutaneous tumours. The most common (in decreasing order of commonality) malignant scalp tumours include basal cell carcinoma (~41%), squamous cell carcinoma (~17%), cutaneous metastases, adnexal tumours, angiosarcomas, and lymphomas.
The causes of both benign and malignant scalp tumours are varied and can depend on the underlying tissue of origin and associated co-morbidities. Scalp tumours can arise from cells in both the skin (epidermis and dermis) and deeper tissue layers. It may also originate from other cells in the body due to metastases.
Both benign and malignant scalp tumours can occur elsewhere on the body but may have different physical features. The exact features displayed are dependent on the originating site and cells of the tumour, summarised in Table 1 and 2.
Keratinocytes |
Firm, flesh-coloured or yellow papules/nodules which may have a central punctum that exudes foul smelling debris. |
||
Keratinocytes |
Firm dough-like lumps consisting of epidermal/dermal tissue components. |
||
Keratinocytes |
Flat or raised lesions with a stuck-on appearance, variable coloration and diameter. Commonly seen in adults over 60 years of age. |
||
Melanocytes |
Flat or raised localized proliferation of melanocytes. Higher propensity for scalp variants to display dysplastic histological features. |
||
Melanocytes |
Flat or raised localized proliferation of spindle shaped or ovoid naevus cells in the dermis. |
||
Hair follicles |
Keratin filled nodules derived from the outer hair root sheath and lacking a central punctum. |
||
Hair follicles |
Skin coloured or purplish irregular papules derived from hair matrix cells which commonly become hard and bony due to calcification. |
||
Sebaceoma |
Sebaceous glands |
Sebaceous cell proliferations that manifest as skin coloured or yellow nodules originating from deeper in the skin than sebaceous adenomas. |
|
Sebaceous adenoma |
Sebaceous glands |
A more superficially located form of sebaceomas manifesting as skin coloured or yellow papules or nodule |
|
Apocrine/eccrine glands |
Apocrine or eccrine derived skin coloured or blue cysts which may arise as solitary multiple lesions. Typically seen on the scalp or face – particularly the eyelid margins (Moll gland cysts). |
||
Apocrine/eccrine glands |
Firm skin coloured or yellow papules millimetres in diameter and commonly occurring in clusters. |
||
Apocrine/eccrine glands |
Papules, plaques, or nodules derived from the epithelial terminal duct which histologically differentiate into poroid (glandular duct) cells. |
||
Adipocytes |
Smooth round collection of subcutaneous fat with a rubbery texture to palpation. |
||
Vascular |
Bright red, blue or flesh-coloured, non-tender and non-pulsatile papules/plaques representing a vascular malformation in the dermis or subcutaneous tissue. |
||
Vascular |
Infantile haemangiomas representing vascular malformations in the lower dermis or subcutaneous tissue. |
||
Vascular |
Skin coloured, blue or purple swellings of variable size that represent malformed veins and are a form of vascular naevi. |
||
Lymphatic |
Malformed lymph ducts of variable size that are a form of vascular naevi and most prominent in infancy or childhood |
||
Myocytes |
Proliferations of myocytes which can develop from both smooth and skeletal muscle. Typically present as firm, smooth, and tender hyperpigmented or red-brown nodules. |
||
Collagen(fibrous)/ histiocytes |
Solitary, firm papules or nodules of variable coloration that may dimple on pinching. |
||
Collagen(fibrous)/ histiocytes |
A growth of fibrous tissue that develops as part of wound healing processes. Typically begins as red and prominent before becoming flat and pale. |
||
Collagen(fibrous)/ histiocytes |
Firm smooth fibrous tissue that characteristically extends beyond the site of the precipitating injury. |
||
Collagen(fibrous)/ histiocytes |
Firm or rubbery circular nodules formed from the proliferation of myofibroblasts in the dermis or subcutaneous tissue. |
||
Neural |
Well circumscribed soft or firm growths derived from Schwann cells, fibroblasts, mast cells, and vascular components of underlying nerves. Occurs in association with Café-au-lait macules in Neurofibromatosis 1 (NF1). |
||
Neural |
Smooth, soft, and solitary skin-coloured or yellow papules or nodules originating in the dermis or subcutaneous tissue and derived from Schwann cells forming the myelin sheath of nerves. |
||
Haematologic |
Overactive accumulation of Langerhans cells in the epidermis with a spectrum of clinical manifestations typically seen in childhood or adolescence. |
||
Haematologic |
Unprovoked histiocyte proliferation disorder characterized by massive cervical lymphadenopathy. |
||
Haematologic |
A non-Langerhans cell histiocytosis that typically manifests as domed red-brown or yellow papules or nodules in children or adolescents. |
||
Keratinocytes |
A malignant proliferation of keratin producing cells extending beyond the epidermis. |
||
Keratinocytes |
Also known as Bowen’s disease. |
||
Keratinocytes |
A malignant proliferation of keratin producing cells extending beyond the epidermis. |
||
Keratinocytes |
Rapidly growing firm circular nodule with a keratin core. A variant of squamous cell carcinoma. |
||
Melanocytes |
Indistinct presentation of variously pigmented or nonpigmented lesions derived from a malignant proliferation of melanocytes. |
||
Hair follicles |
Keratinized nodules or cysts derived from outer hair root sheath cells with low potential for metastasis. May develop from trichilemmal cysts. |
||
Hair follicles |
A low grade adnexal carcinoma derived from hair matrix cells that may manifest as skin coloured or purplish irregular papules. |
||
Sebaceous glands |
A form of adnexal carcinoma where the cells demonstrate a differentiation into sebaceous cells. |
||
Sweat glands |
An adenocarcinoma derived from apocrine glands. Lesions may manifest as ulcerating or bleeding nodules but otherwise have an indistinct presentation and are usually diagnosed histologically. |
||
Sweat glands |
An adenocarcinoma derived from eccrine (sweat) glands. As with apocrine carcinomas, lesions have a nondescript appearance but may appear as ulcerating or bleeding nodules that are diagnosed histologically. |
||
Atypical lipomatous tumour |
Adipocytes |
Malignant proliferation of adipocytes that rarely develops in the skin but can resemble an enlarging lipoma. Differentiated from liposarcoma on the basis of histology findings. |
|
Adipocytes |
Malignant proliferation of adipocytes that can present identically to an atypical lipomatous tumour or an enlarging lipoma. |
||
Vascular |
Aggressive tumours that uncommonly develop from endothelial cells in blood (haemangiosarcoma) or lymphathic (lymphangiosarcoma) vessels |
||
Myocytes |
Malignant proliferation of smooth muscle cells that may also develop in the dermis or subcutaneous tissue. |
||
Collagen(fibrous)/ histiocytes |
Slow growing tumours derived from collagen that develop in the dermis. |
||
Fibrosarcoma |
Collagen(fibrous)/ histiocytes |
Malignant proliferation of spindled fibroblasts or myofibroblasts which are typically firm and spherical but otherwise nondescript in appearance. Generally carries a poor prognosis. |
|
Neural |
Aggressive tumours with high metastatic potential, thought to be derived from pressure receptors (Merkel cells) in the skin. Approximately 80% of cases are found to have concomitant Merkel cell polyomavirus. The most common site of development is the head and neck region. Scalp lesions are typically larger and have an even higher risk of metastasis. |
||
Malignant peripheral nerve sheath tumour |
Neural |
Proliferations of one or more types of cells that compose peripheral nerve sheaths – typically originate from perineural or endoneurial fibroblasts. Associated with plexiform neurofibromas. |
|
Haematologic |
A malignant proliferation of lymphocytes. Multiple cutaneous and non-cutaneous variants exist. The most commonly occurring variants on the scalp include primary cutaneous follicle centre and/or marginal zone lymphomas. |
||
Metastases |
Various |
Secondary malignant proliferations that develop from the spread of a primary malignancy beyond its site of origin.
|
|
Some scalp tumours may be diagnosed through a clinical examination alone. A biopsy or radiologic workup may be necessary to confirm the diagnosis.
Treatment options are dependent on the nature of the tumour, anatomical location, and underlying diagnosis. Given that malignant scalp tumours carry a worse prognosis than lesions in other anatomic areas, radical surgical excision is more likely to be recommended. Excision can be complicated by the relative lack of scalp skin mobility. Mohs micrographic surgery may be a preferable option in these cases.
Other treatments may include:
Malignant scalp tumours tend to carry a worse prognosis than equivalent tumours elsewhere on the body. The exact prognosis is dependent on the specific tumour involved and the degree of invasion beyond the skin. Scalp metastases (either originating from scalp tumours or elsewhere) typically carry a poor prognosis.